Canagliflozin Amputation Risk Calculator
This tool estimates your relative risk of amputation if taking canagliflozin (Invokana) based on your medical history. According to clinical studies, patients with multiple risk factors have a higher chance of amputation. Remember, this is an estimation tool and should not replace professional medical advice.
Risk Factors
Your Amputation Risk Assessment
Recommended actions:
- Check your feet daily
- Wear properly fitted shoes
- Report any sores or changes immediately
- Consider discussing alternatives with your doctor
When you’re managing type 2 diabetes, choosing the right medication isn’t just about lowering blood sugar. It’s about weighing every possible risk-especially when one of them could mean losing a toe, or worse. Canagliflozin, sold under the brand name Invokana, is one of those drugs that came with a warning that shook the diabetes world. In 2017, the FDA slapped a boxed warning on it: higher risk of leg and foot amputations. That warning was pulled two years later. But the risk? It didn’t disappear. It just got quieter.
What the Data Actually Shows
The biggest study that raised the alarm was the CANVAS Program, which tracked over 10,000 people with type 2 diabetes and heart disease over several years. Those taking canagliflozin had about twice the rate of amputations compared to those on placebo. For the 300 mg dose, it was 5.5 amputations per 1,000 people each year-up from 2.8 in the placebo group. That’s not a small jump. And while most of these were minor-like toe or metatarsal amputations-20% were major, above the ankle. Here’s the twist: this risk doesn’t seem to apply to all drugs in the same class. Empagliflozin (Jardiance) and dapagliflozin (Farxiga) didn’t show the same pattern in their own large trials. In fact, dapagliflozin’s trial suggested a possible reduction in amputation risk. A 2023 meta-analysis confirmed it: among SGLT2 inhibitors, only canagliflozin had a statistically significant link to amputation. That’s not a coincidence. It’s a signal.Why Only Canagliflozin?
Scientists aren’t 100% sure why, but they have strong theories. Canagliflozin lowers blood pressure and body weight more than other drugs in its class. That might sound good-until you’re someone with poor circulation in your legs. If your arteries are already narrowed from diabetes, a sudden drop in blood pressure might reduce blood flow even further. Add in nerve damage (common in diabetes), and you’ve got a perfect storm: you can’t feel a sore developing, and your body can’t heal it fast enough. The numbers tell the story. One analysis found that for every 556 people treated with canagliflozin for a year, one extra amputation happened. That’s rare-but not rare enough to ignore, especially if you’re already at risk.Who’s Most at Risk?
This isn’t a risk for everyone on canagliflozin. It’s focused. If you have any of these, your risk goes up:- History of foot ulcers or prior amputation
- Diabetic neuropathy (nerve damage causing numbness)
- Peripheral artery disease (PAD)-poor blood flow to the legs
- Smoking
- Absent foot pulses (a sign of blocked arteries)
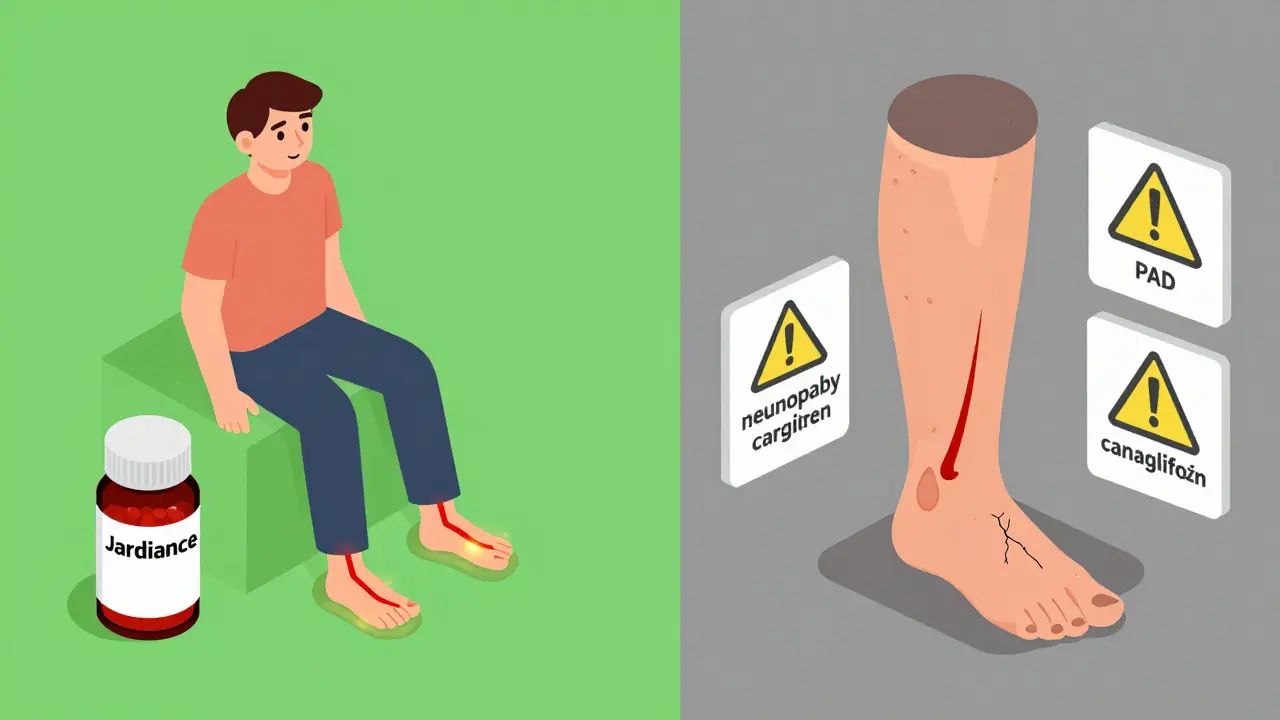
What Doctors Are Doing Now
The FDA removed the boxed warning in 2020-not because the risk vanished, but because the benefits in kidney and heart protection outweighed the risk for many. Still, the prescribing label still says: monitor for pain, sores, or infections in the feet and legs. In 2024, the American Diabetes Association updated its guidelines to recommend an ankle-brachial index (ABI) test before starting canagliflozin. This simple, non-invasive test measures blood pressure in your ankles compared to your arms. If the ratio is below 0.9, it means your leg arteries are narrowed. That’s a red flag. Don’t start canagliflozin without addressing it first. Clinics are also stepping up foot checks. Instead of a quick glance at your toes once a year, doctors are now expected to examine your feet at every visit. They’re checking for cracks, calluses, swelling, and loss of sensation. And they’re handing out printed guides-68% of new prescriptions in 2023 came with a warning sheet about foot care, up from 42% in 2017.Real Stories, Real Consequences
On PatientsLikeMe, nearly 7% of canagliflozin users reported foot problems. A Reddit user shared that after 18 months on Invokana, a non-healing ulcer led to a toe amputation. His endocrinologist switched him to Jardiance immediately. Another user, on the same platform, said she’s been on it for three years with no issues and her A1c dropped from 8.5% to 6.2%. The FDA’s own database shows a stark difference: 1,892 amputation reports for canagliflozin versus just 327 for empagliflozin-despite far more people taking the latter. The reporting odds ratio? Nearly 18 times higher. That’s not noise. That’s a pattern.What You Can Do
If you’re on canagliflozin, here’s what you need to do:- Check your feet every single day. Look between your toes. Feel for warmth, swelling, redness.
- Report any sore, cut, blister, or pain-even if it doesn’t hurt. Nerve damage hides pain.
- Wear properly fitted shoes. No barefoot walking, even indoors.
- Don’t ignore dry, cracked skin. Use moisturizer daily.
- Ask your doctor for an ABI test if you’ve never had one.
- If you smoke, quit. Smoking kills circulation faster than almost anything.
The Bigger Picture
Despite the concerns, canagliflozin still made $1.87 billion in sales in 2023. It’s still prescribed. It still works. For someone without foot problems, with strong circulation, and who needs to protect their kidneys and heart, it can be life-changing. But it’s not for everyone. And it’s not a drug you should take without knowing the risks-and how to prevent them. The goal isn’t to scare you off. It’s to make sure you’re not blindsided. The FOOT-STEP trial, set to finish in 2026, is testing whether structured foot care programs can cut the amputation risk in half. Janssen is even testing a new extended-release version of the drug, hoping to reduce peak blood levels that might be contributing to the problem. For now, the message is clear: canagliflozin is a tool. Like any tool, it’s powerful when used right-and dangerous when used carelessly. Know your risks. Monitor your feet. Ask questions. And if your doctor doesn’t bring up foot health, bring it up yourself.Is canagliflozin still safe to take?
Yes-for people without pre-existing foot or circulation problems. The benefits for heart and kidney protection can outweigh the risk. But it’s not safe for everyone. If you have a history of foot ulcers, nerve damage, or poor leg circulation, your doctor should consider alternatives like Jardiance or Farxiga.
Why was the FDA boxed warning removed?
The FDA removed the boxed warning after reviewing more data, including the CREDENCE trial, which showed strong kidney and heart benefits in people with diabetic kidney disease. The agency concluded that for many patients, the benefits outweighed the risks. But the warning about amputation remains in the prescribing information.
Do all SGLT2 inhibitors cause amputation risk?
No. Only canagliflozin has shown a consistent, statistically significant increase in amputation risk across multiple studies. Empagliflozin (Jardiance) and dapagliflozin (Farxiga) have not shown this risk in large trials, and some data even suggest a lower risk.
How often should I check my feet?
Every single day. Look for cuts, blisters, redness, swelling, or changes in skin color. Feel for warmth or cold spots. If you have numbness from nerve damage, you can’t rely on pain to warn you. Daily checks are non-negotiable.
What should I do if I notice a foot sore?
Call your doctor or podiatrist immediately. Don’t wait. Don’t try to treat it yourself. Diabetic foot ulcers can turn into serious infections in days. Early intervention is the only way to avoid amputation.
Can I switch from canagliflozin to another SGLT2 inhibitor?
Yes, and many people do. If you have risk factors for foot problems, switching to Jardiance or Farxiga is a common and safe choice. Both drugs offer similar heart and kidney protection without the same amputation risk. Talk to your doctor about making the switch.
Adam Rivera
January 13, 2026 AT 08:22Man, I’ve been on Jardiance for two years now and my feet have never felt better. My doc told me to ditch Invokana after my ABI came back at 0.87 - no joke, I didn’t even know what that was until he explained it. Now I check my toes every night like it’s my job. Best habit I’ve ever picked up.
Acacia Hendrix
January 14, 2026 AT 11:54It’s not merely a pharmacokinetic discrepancy - it’s a class-wide epiphenomenon misattributed due to off-target SGLT1 inhibition in canagliflozin’s molecular architecture. The 300mg dose exhibits a disproportionate renal tubular reabsorption profile, leading to hemodynamic instability in vulnerable microcirculatory beds. This is why empirical clinical guidelines must be stratified by endothelial resilience indices, not just HbA1c thresholds.
lucy cooke
January 15, 2026 AT 11:35They removed the boxed warning because the pharmaceutical lobby whispered sweet nothings in the FDA’s ear. But the amputations? They didn’t vanish - they just got buried under layers of corporate PR and ‘benefit-risk ratios.’ We’re not patients here. We’re data points in a spreadsheet. And someone’s profit margin just got a little fatter.
John Pope
January 16, 2026 AT 06:00Look, I get it - you wanna feel like you’re doing something for your health, but let’s be real. You’re not gonna check your feet every day. You’re gonna forget. You’re gonna think ‘it’s just a blister’ and then boom - six weeks later you’re in the hospital with a gangrenous toe. I’ve seen it. My uncle lost both feet. He didn’t even know his socks were too tight until it was too late. This isn’t about meds - it’s about systemic neglect. The system doesn’t care if you live or die, as long as you keep paying for the script.
And don’t even get me started on ‘alternative SGLT2 inhibitors.’ Jardiance? Farxiga? Same damn company, same damn playbook. They just tweaked the molecule so the FDA didn’t have to slap another warning on it. It’s all smoke and mirrors. You think they care about your feet? They care about your co-pay.
Kimberly Mitchell
January 17, 2026 AT 00:31Why are we even debating this? If you have neuropathy and PAD, you shouldn’t be on any SGLT2 inhibitor. Period. The fact that doctors still prescribe this without mandatory ABI screening is malpractice. And the patients who say ‘I’m fine’? They’re either lying or delusional. Nerve damage doesn’t announce itself with a scream - it whispers until you’re already missing toes.
Randall Little
January 18, 2026 AT 10:48So let me get this straight - the drug that causes amputations has a 18x higher reporting rate than its peers, but the FDA removed the boxed warning because… benefits outweigh risks? Who’s benefiting? The patient? Or the stockholders who got a $1.87 billion payday? This isn’t medicine. It’s capitalism with a stethoscope.
John Tran
January 18, 2026 AT 11:12okay so i was on canagliflozin for like 2 years and i never had any foot issues but my cousin? dude lost his big toe and he was like 45 and he smoked and had type 2 for 15 years and his doc just gave him invokana like it was tylenol. i mean like… why? why not just test his ankles first? i read this article and i was like holy crap i knew something was off but i thought it was just my imagination. now i’m scared to even take my own meds. and then i saw some guy on reddit saying he’s fine? yeah but what if you’re the 1 in 500? you’re not gonna know until it’s too late. my cousin cried in the hospital and he couldn’t walk for months. now he uses a cane. and the worst part? he still takes the drug. his doc says ‘you’re fine now’ like that means anything. it doesn’t. it doesn’t mean a damn thing.
mike swinchoski
January 19, 2026 AT 21:08Why are we still talking about this? Just don’t take it if you’re old or have foot problems. Duh. It’s not rocket science.
Lethabo Phalafala
January 21, 2026 AT 07:29I’m from South Africa, and here, most people with diabetes don’t even have access to podiatrists. We don’t get ABI tests. We don’t get foot exams. We get a script and a prayer. So when I read about this, I cried. Not because I’m scared - because I know so many people who’ve lost limbs and no one ever told them. This isn’t just about one drug. It’s about who gets cared for - and who gets left behind. If you’re reading this and you have access to doctors, check your feet. And if you can, help someone who can’t.
Lance Nickie
January 22, 2026 AT 17:05Canagliflozin is fine. The real problem? People don’t wear socks.