Why Generic Drug Names Matter More Than You Think
Imagine a patient in Germany gets prescribed salbutamol. In the U.S., the same drug is called albuterol. Same pill. Same effect. But different names. One wrong letter, one misheard syllable - and you could be giving the wrong dose, the wrong drug, or worse. This isn’t a hypothetical. Medication errors caused by confusing drug names cost the U.S. healthcare system about $2.4 billion every year. That’s why systems like USAN and INN exist - not for bureaucracy, but to save lives.
What Are USAN and INN?
USAN stands for United States Adopted Names. It’s the official system the U.S. uses to assign nonproprietary names to drugs. Think of it as the American dictionary for drug substances. It’s managed by the USAN Council, a group made up of the American Medical Association, the U.S. Pharmacopeia, and the American Pharmacists Association. They’ve been doing this since 1964.
INN means International Nonproprietary Name. Run by the World Health Organization since 1950, it’s the global standard. Every country that follows WHO guidelines uses INN names. That’s most of them - including Canada, the UK, Australia, and nearly every nation in Europe and Asia.
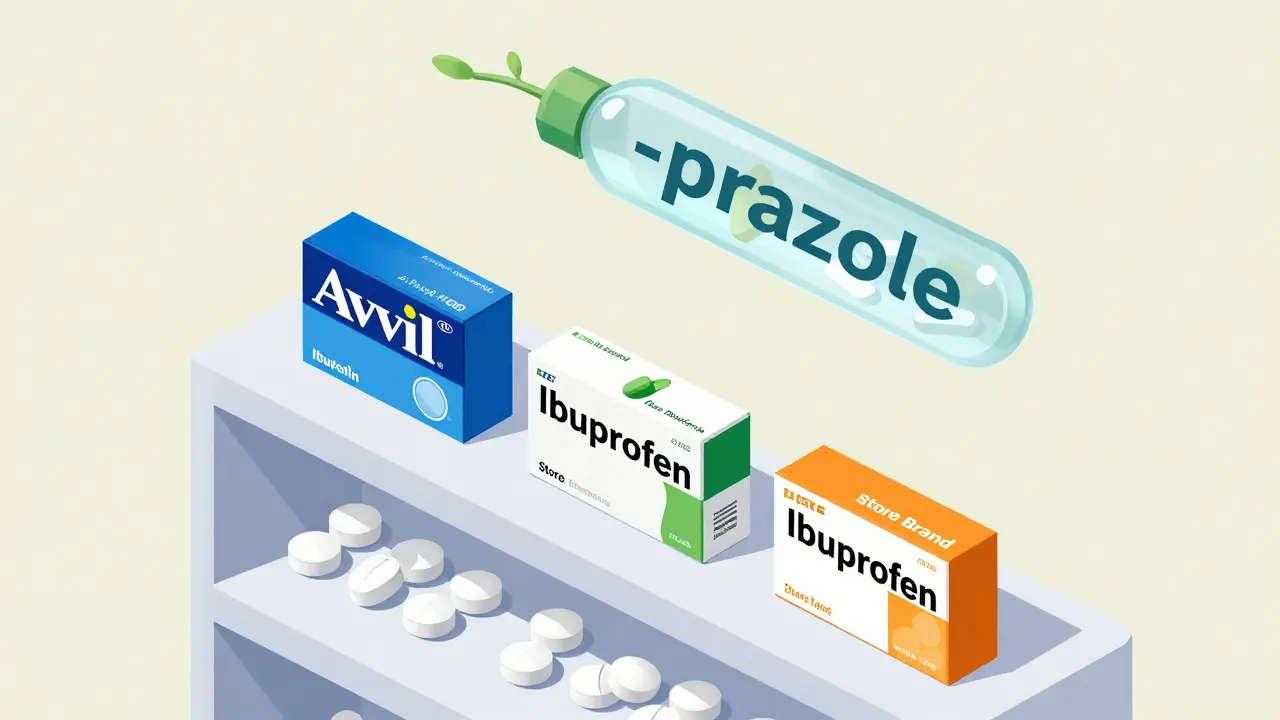
Neither system lets companies trademark these names. That’s key. The name belongs to the public. Any manufacturer can use it. That’s why you see ibuprofen sold under Advil, Motrin, or a store brand - the generic name is the same everywhere.
The Stem System: How Names Reveal What a Drug Does
Here’s the clever part: generic drug names aren’t random. They follow a pattern. The ending - the stem - tells you the drug’s class. The front part - the prefix - is mostly made up to sound unique.
Take -mab. That’s for monoclonal antibodies. So if you see rituximab, adalimumab, or trastuzumab, you instantly know it’s a biologic drug targeting specific cells in the body. But look closer:
- -ximab = chimeric (part mouse, part human)
- -zumab = humanized (mostly human)
- -umab = fully human
Same family. Different details. That’s the power of stems.
Other common stems:
- -prazole = proton pump inhibitors (omeprazole, pantoprazole)
- -statin = cholesterol-lowering drugs (atorvastatin, rosuvastatin)
- -feron = interferons (interferon alfa-2a)
- -virdine = HIV drugs (zidovudine, lamivudine)
Doctors don’t need to memorize every drug. They just need to know the stem. If a patient says they’re on something ending in -prazole, you know it’s for stomach acid. That’s safety built into the name.
Why Do the U.S. and the Rest of the World Have Different Names?
Most of the time, USAN and INN match. But not always. There are about a dozen common mismatches that cause real confusion.
Here are the big ones:
| USAN Name | INN Name | Drug Class |
|---|---|---|
| Albuterol | Salbutamol | Asthma inhaler |
| Acetaminophen | Paracetamol | Pain reliever |
| Rifampin | Rifampicin | TB antibiotic |
| Hydralazine | Hydralazine | Blood pressure |
| Fluoxetine | Fluoxetine | Antidepressant |
Why do these exist? History. The U.S. had its own naming tradition before global standards took hold. Some names stuck because they were already in use in American hospitals and pharmacies. The WHO didn’t force a change - it was easier to keep both.
But here’s the problem: a nurse in a U.S. hospital might never hear “paracetamol.” A doctor in London might not know “acetaminophen.” If a patient travels or gets records sent internationally, that mismatch can lead to dosing errors or missed treatments.
How a Drug Gets Its Name - The Long Road from Lab to Label
It doesn’t start with marketing teams. It starts in a lab, long before the drug hits the market.
When a pharmaceutical company has a new molecule ready for human testing, they apply to both USAN and INN. They submit up to six name options, ranked by preference. Then the real work begins.
The USAN Council checks:
- Does it sound too similar to another drug? (e.g., Hydrea vs. Hydralazine)
- Is the stem accurate? (Does it fit the drug’s mechanism?)
- Is it easy to pronounce in English?
- Does it have unintended meanings in other languages?
They run automated checks across millions of drug names, medical journals, and trademark databases. If a name is too close to something else - even a brand name - it gets rejected.
Then they send the top choice to WHO’s INN team. WHO does the same checks, but globally. If they agree, the name gets published. If not, they suggest an alternative.
This whole process takes 18 to 24 months. That’s why companies start the naming process early - during Phase 1 clinical trials. If they wait until approval, they risk delays.
And here’s a shocker: about 65% of drugs that get a USAN name never make it to market. The science fails. The trials flop. But the name still exists - just in case someone else picks it up later.
Brand Names vs. Generic Names: What’s the Difference?
Generic names (USAN/INN) are public property. Brand names are owned by companies. That’s why you see:
- Generic: lisinopril
- Brand: Zestril, Prinivil
- Generic: atorvastatin
- Brand: Lipitor
Brand names are marketing tools. They’re catchy, easy to remember, and often tied to logos and ads. But they don’t tell you anything about the drug’s function. “Lipitor” doesn’t hint at cholesterol. “Zestril” doesn’t say “blood pressure.”
Generic names? They do. That’s why doctors often write prescriptions using the generic name - it’s clearer, safer, and cheaper.
But here’s the twist: some brand names become so popular, people forget the generic. “Prozac” is still used instead of “fluoxetine.” “Viagra” instead of “sildenafil.” That’s fine for consumers - but dangerous for medical records. That’s why the FDA insists on using generic names in official documents.

What’s Changing in Drug Naming?
Science is moving faster than naming rules. New drugs aren’t just pills anymore. They’re gene therapies, RNA treatments, antibody-drug conjugates - complex, one-of-a-kind medicines.
Traditional stems like -mab or -prazole don’t fit them. So WHO and USAN are updating their rules.
In 2021, WHO revised monoclonal antibody naming to cover newer types like bispecific antibodies and Fc-engineered versions. They added new suffixes to show these differences.
For RNA drugs, there’s no standard yet. Some companies use -RNA as a suffix. Others don’t. That’s a problem. If a doctor sees a drug ending in -RNA, they might assume it’s similar to another - but it might not be.
Experts warn: if naming doesn’t keep up, we’ll see more errors. The system was built for simple molecules. Now it’s handling living therapies. That’s why both USAN and INN are working with scientists to create new stems - but only when absolutely necessary. They don’t want to overcomplicate things.
Why This All Matters to You
You might never apply for a drug name. But you might take one. Or prescribe one. Or fill a prescription.
If you’re a patient: know your drug’s generic name. Ask your pharmacist. Write it down. If you travel, bring a list of your meds with both brand and generic names.
If you’re a healthcare worker: always check for USAN/INN differences. Don’t assume a name is the same overseas. Use electronic systems that flag mismatches.
If you’re in pharma: don’t cut corners on naming. It’s not a branding exercise. It’s a safety protocol. A bad name can cost lives - and millions in recalls.
Generic drug names aren’t boring labels. They’re coded messages. They’re the silent safety net in a system full of risks. And they’re working - even if you never notice them.
What’s Next for Drug Naming?
Global harmonization is the goal. More countries are adopting INN as their official standard. The U.S. still uses USAN, but the FDA accepts INN names for imported drugs. The gap is shrinking.
One day, there may be just one global nonproprietary name. But until then, we live with two. And that’s why understanding both systems isn’t just for chemists - it’s for anyone who uses medicine.
Helen Leite
January 22, 2026 AT 18:27Darren Links
January 22, 2026 AT 23:26Izzy Hadala
January 23, 2026 AT 13:59Elizabeth Cannon
January 24, 2026 AT 13:46Gina Beard
January 24, 2026 AT 21:50Don Foster
January 26, 2026 AT 14:55Phil Maxwell
January 26, 2026 AT 23:42Patrick Gornik
January 27, 2026 AT 21:22Sushrita Chakraborty
January 28, 2026 AT 18:37Josh McEvoy
January 29, 2026 AT 19:58Heather McCubbin
January 31, 2026 AT 07:07Shanta Blank
February 1, 2026 AT 11:23