When you take a medication like levothyroxine or tacrolimus, the difference between taking the right dose and the wrong one isn’t just a matter of feeling off-it can mean the difference between staying healthy and ending up in the hospital. These are called NTI drugs-narrow therapeutic index drugs. That means there’s a tiny window between the dose that works and the dose that harms you. A 5% change in blood levels might be fine for most pills, but for NTI drugs, it can trigger serious side effects or make the drug stop working entirely.
What Makes a Drug an NTI Drug?
NTI drugs have a very small safety margin. Take warfarin, for example. It’s used to prevent blood clots, but if your blood levels go too high, you risk dangerous bleeding. Too low, and you could get a stroke. Its therapeutic index is only about 2 to 4-meaning the toxic dose is barely double the effective dose. The same goes for digoxin, used for heart failure. A few nanograms per milliliter can mean the difference between control and toxicity.
The FDA doesn’t publish a full list of NTI drugs, but they’ve clearly identified key ones: carbamazepine, phenytoin, lithium, cyclosporine, theophylline, and tacrolimus. These aren’t just any generics. They’re drugs where even minor changes in how they’re made-like the filler, coating, or release rate-can affect how your body absorbs them.
Why Generic Switching Gets Complicated
When a pharmacy fills your prescription, they often pick the cheapest generic available. That’s fine for most medications. But with NTI drugs, switching between different generic manufacturers can cause real problems-even if all versions are technically "bioequivalent."
The FDA requires generics to be within 80-125% of the brand drug’s absorption rate. But for NTI drugs, that’s too loose. Since 2018, the FDA has tightened those rules. For some NTI drugs, the acceptable range is now 90-111%, and for others, it’s even tighter: 95-105% for the active ingredient’s strength. That sounds precise, but here’s the catch: bioequivalence studies are done on healthy volunteers, not people with kidney failure, liver disease, or complex drug interactions.
A 2019 study comparing different generic versions of tacrolimus found that one brand had 93% of the active ingredient, another had 110%. That’s a 17% difference between two pills both labeled the same. The FDA says that’s still within acceptable limits. But for a kidney transplant patient on a strict regimen, that variation can mean the difference between organ rejection and stability.
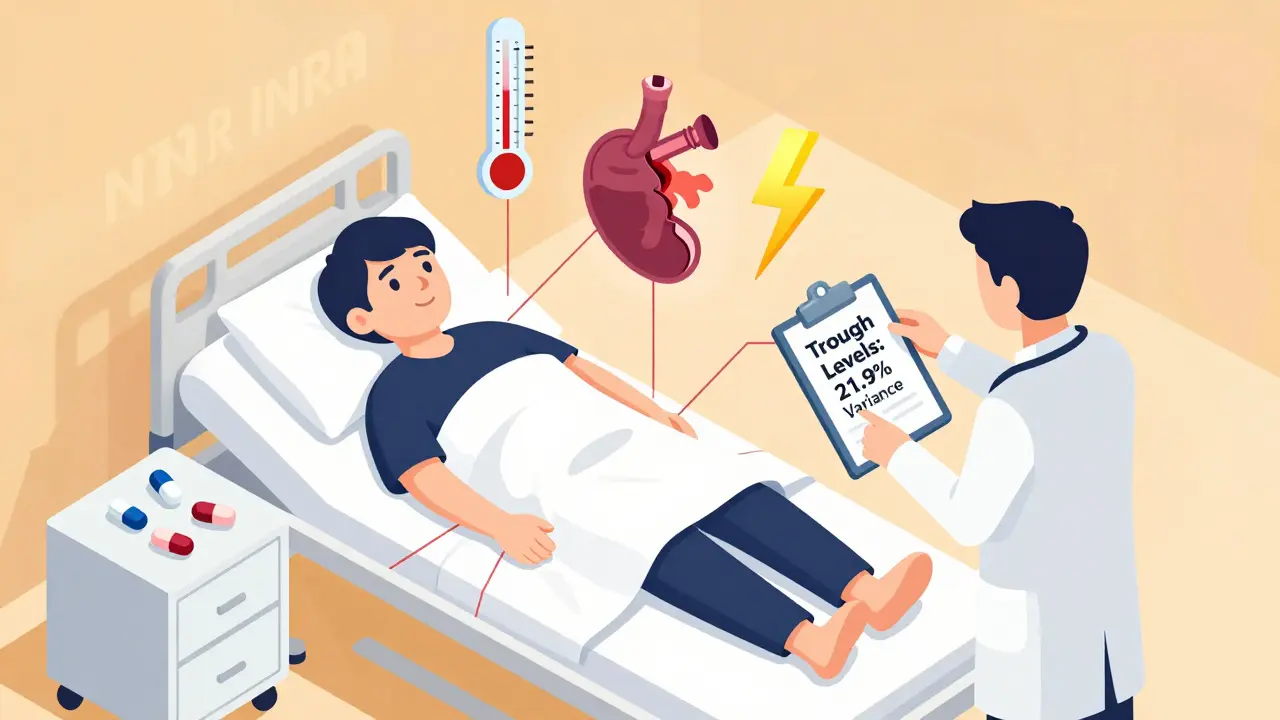
One study showed that when patients switched from one generic tacrolimus to another, their blood levels varied by 21.9% on average. That’s not a typo. Nearly one in five patients saw a big swing in drug levels-enough to trigger rejection or toxicity. The same pattern shows up with cyclosporine, where switching formulations led to a 15.3% higher rate of acute rejection in transplant patients.
Real-World Stories vs. Clinical Data
The FDA says generic NTI drugs are therapeutically equivalent. And statistically, they’re right. Large studies on levothyroxine show no meaningful difference in TSH levels between brand-name Synthroid and generics. One 2021 FDA analysis found mean TSH levels of 2.12 for Synthroid and 2.15 for generics-statistically identical.
But here’s what those numbers don’t tell you: some patients still have problems. A 2019 survey of pharmacists found that 63% had received complaints from patients or doctors after switching between generic manufacturers of NTI drugs. Patients report sudden fatigue, heart palpitations, anxiety, or seizures-symptoms that didn’t exist before the switch.
The Epilepsy Foundation gets calls every week from families whose child had a breakthrough seizure after a pharmacy switched their carbamazepine from one generic to another. The FDA’s bioequivalence studies say it shouldn’t happen. But it does. And when it does, it’s not a statistical anomaly-it’s a real person in crisis.
Why Pharmacists Are Caught in the Middle
Pharmacists are trained to save money and follow the law. In most states, they’re required to substitute generics unless the doctor says "dispense as written." But 27 states have special rules for NTI drugs. In those states, pharmacists can’t switch NTI generics without the prescriber’s approval-especially for drugs like phenytoin or levothyroxine.
Why? Because even if the science says it’s safe, the risk isn’t zero. And when the stakes are high-seizures, transplant rejection, strokes-many doctors and patients don’t want to gamble.
A 2019 survey found that 82% of pharmacists almost always substituted generics for NTI drugs on initial prescriptions. But 94% said they believed they were safe, and 87% thought they were effective. That’s a huge disconnect: pharmacists trust the science, but they’ve also seen the fallout.
What Patients Should Do
If you’re on an NTI drug, here’s what you need to know:
- Ask your doctor to write "dispense as written" or "no substitution" on your prescription if you’ve had stability with a specific brand or generic.
- Keep a list of the manufacturer name and color of your pills. If your pill looks different, ask why.
- Don’t assume "same drug = same effect." Even small changes in fillers or coatings can affect absorption, especially if you have digestive issues or take multiple medications.
- Get your blood levels checked after any switch. For warfarin, that means INR. For lithium, it’s serum levels. For tacrolimus, it’s trough levels. Don’t wait for symptoms.
- If you feel off after a switch-fatigue, dizziness, tremors, mood changes-don’t brush it off. Call your doctor. It might be the drug.
The Bigger Picture: Who Benefits?
Drug manufacturers change their formulas too. Brand-name companies tweak coatings, release mechanisms, or inactive ingredients-all without FDA reapproval. The American Medical Association pointed out in 2007 that brand-name changes happen "not infrequently." So why is switching from one generic to another seen as risky, but switching from brand to brand is considered normal?
The answer isn’t about science. It’s about control. Brand-name companies profit from keeping patients locked into their version. Generic companies profit from volume. Patients and doctors are stuck in the middle.
There’s no perfect system. But we know this: for NTI drugs, consistency matters more than cost. A 2023 report from the American Society for Clinical Pharmacology and Therapeutics warned that multiple switches increase the risk of serious adverse events. That’s not speculation-it’s evidence.
So if you’re on an NTI drug, and you’ve found a version that keeps you stable-stick with it. Ask your doctor to lock it in. If your pharmacy tries to switch, ask them to call your prescriber. Your health isn’t a commodity. It’s your life.
What’s Next for NTI Drug Regulation?
The FDA is still studying this. Their Post-market Research and Perceptions of Generic NTI Drugs initiative is tracking real-world outcomes. Early data shows no major safety issues for most patients. But they’re also aware of the gaps.
Some experts are calling for a new category: "non-interchangeable generics" for NTI drugs. That means even if two generics are bioequivalent, they can’t be swapped without a doctor’s order. A few states have already tried this. It’s not perfect, but it gives doctors and patients control.
For now, the safest path is simple: know your drug, track your levels, and speak up if something changes. Your body knows when something’s off-even if the lab results say everything’s fine.
david jackson
December 27, 2025 AT 11:07Okay, so let me get this straight - the FDA says two generics are "bioequivalent" if they’re within 80-125% of the brand, but for drugs like tacrolimus, a 17% swing between two pills labeled the same can literally mean your new kidney gets rejected? That’s not science, that’s Russian roulette with a prescription pad. And don’t get me started on how these studies are done on healthy 22-year-olds who’ve never had a kidney in their life, while the actual patients are 65-year-olds on ten other meds, with cirrhosis, diabetes, and a history of vomiting after eating toast. The system isn’t broken - it’s been designed this way to save pennies while people’s lives hang in the balance. I’ve seen it. My cousin’s transplant team had to switch her from one generic to another because of insurance, and within three weeks, her creatinine spiked, her white blood cell count went nuclear, and she spent a month in the hospital. They didn’t even test her levels before the switch. No one does. Because it’s cheaper to fix the damage than prevent it.
Prasanthi Kontemukkala
December 28, 2025 AT 06:26This is such an important topic, and thank you for laying it out so clearly. I work in a clinic in India where many patients rely on generics, and I’ve seen how small changes in pill color or shape cause panic - even when the drug is technically the same. The real issue isn’t the science, it’s the lack of communication. Patients don’t know to ask about the manufacturer, and pharmacists are under pressure to cut costs. Maybe we need a simple sticker on the bottle - like "This batch is from [Manufacturer X]" - so patients can track what works for them. Small change, huge impact.
Bryan Woods
December 29, 2025 AT 03:28The data presented here is compelling, and the clinical implications are significant. While the FDA’s bioequivalence standards are statistically sound, they fail to account for inter-individual variability in pharmacokinetics, particularly in populations with comorbidities. The 2019 tacrolimus study cited demonstrates a clear clinical disconnect between regulatory thresholds and real-world outcomes. It is therefore reasonable to advocate for stricter interchangeability criteria for NTI drugs, particularly in transplant and neurology populations where therapeutic margins are minimal.
Ryan Cheng
December 30, 2025 AT 08:40Just wanted to add - if you’re on an NTI drug, don’t just rely on your doctor to catch it. Be your own advocate. Keep a little notebook with the name of the pill, the color, the logo, and the pharmacy. I switched from one generic to another and felt like a zombie for two weeks. My doctor said, "Your labs are fine." But I knew something was off. I showed him my notes - the pill was different, I’d never seen that logo before. He called the pharmacy. Turned out they switched without telling anyone. Now he writes "DO NOT SUBSTITUTE" on everything. It’s not paranoia. It’s survival.
wendy parrales fong
January 1, 2026 AT 05:43I love how you said "your body knows when something’s off" - that’s so true. I’m a nurse and I’ve had patients tell me, "I just don’t feel right," and the labs say perfect. But you know what? They’re right. Sometimes the numbers don’t tell the whole story. Your body remembers. If you feel weird after a switch, trust that. Don’t wait for the hospital. Call your doc. Text them. Send a voice note. You deserve to feel okay. Your health isn’t a spreadsheet.
Jeanette Jeffrey
January 2, 2026 AT 17:18Oh please. Let’s not pretend this is some grand conspiracy. People are just dramatic. If your thyroid levels are normal, why are you still tired? Maybe you’re depressed. Maybe you eat too much sugar. Maybe you’re just lazy. The FDA doesn’t lie. If your blood work is clean, stop blaming the pill and start blaming yourself. Also, why are you so obsessed with pill colors? Are you a child? Grow up.
Zina Constantin
January 3, 2026 AT 21:52As someone who grew up in a country where generics are the only option, I’ve seen how this plays out. In the U.S., people have the luxury of complaining about switching. In Nigeria, India, or Peru, patients don’t even know what manufacturer made their pill. They just take it. But here’s the thing - even in places with no oversight, people find ways to survive. They trade stories. They share pill photos. They remember which batch made them feel okay. Maybe the real solution isn’t more regulation - it’s community. Knowledge sharing. People helping each other notice the little things before the big things happen.
Dan Alatepe
January 4, 2026 AT 07:58Bro. I took carbamazepine for 8 years. One day, the pharmacy gave me a blue oval instead of the white triangle. I started having seizures. Like, full-on, floor-rolling, foaming-at-the-mouth seizures. I called the pharmacy. They said, "It’s the same drug." I said, "Then why did my brain just try to escape my skull?" They didn’t know what to say. I went to the ER. They checked my levels. My drug was 30% lower. I cried in that hospital bed. Not because I was scared - because I realized no one cares until you almost die. Now I take my pills like they’re sacred. I even take a pic of the bottle before I leave the pharmacy. I’m not crazy. I’m just tired of being a guinea pig.
Angela Spagnolo
January 5, 2026 AT 02:14I... I just... I’ve been on levothyroxine for 12 years, and I’ve switched generics so many times, I’ve lost count. I’ve had panic attacks, hair falling out, heart racing... and every time I go to my doctor, they say, "Your TSH is perfect." But I’m not perfect. I’m exhausted. I’m sad. I’m... I’m not me. I don’t know what to do. I’ve tried everything. I even wrote a letter to the FDA. They never replied. I just... I just want to feel normal again. I don’t know if anyone else gets this. But if you do... I see you.
Sarah Holmes
January 6, 2026 AT 16:29This entire post is a dangerous oversimplification of pharmacoeconomics and a disservice to public health policy. The FDA’s standards are based on decades of rigorous, peer-reviewed clinical trials. To suggest that bioequivalence is meaningless is to undermine the very foundation of evidence-based medicine. The anecdotal reports you cite are statistically insignificant and reflect confirmation bias, not causality. Patients who report symptoms post-switch are often influenced by the nocebo effect - a well-documented psychological phenomenon. Blaming manufacturers and pharmacists for patient anxiety is irresponsible. The real issue is patient education - not regulatory rollback.
Jay Ara
January 7, 2026 AT 06:28