Tag: bioequivalence
Reassurance from Research: What Clinical Studies Say About Brand-to-Generic Drug Switches
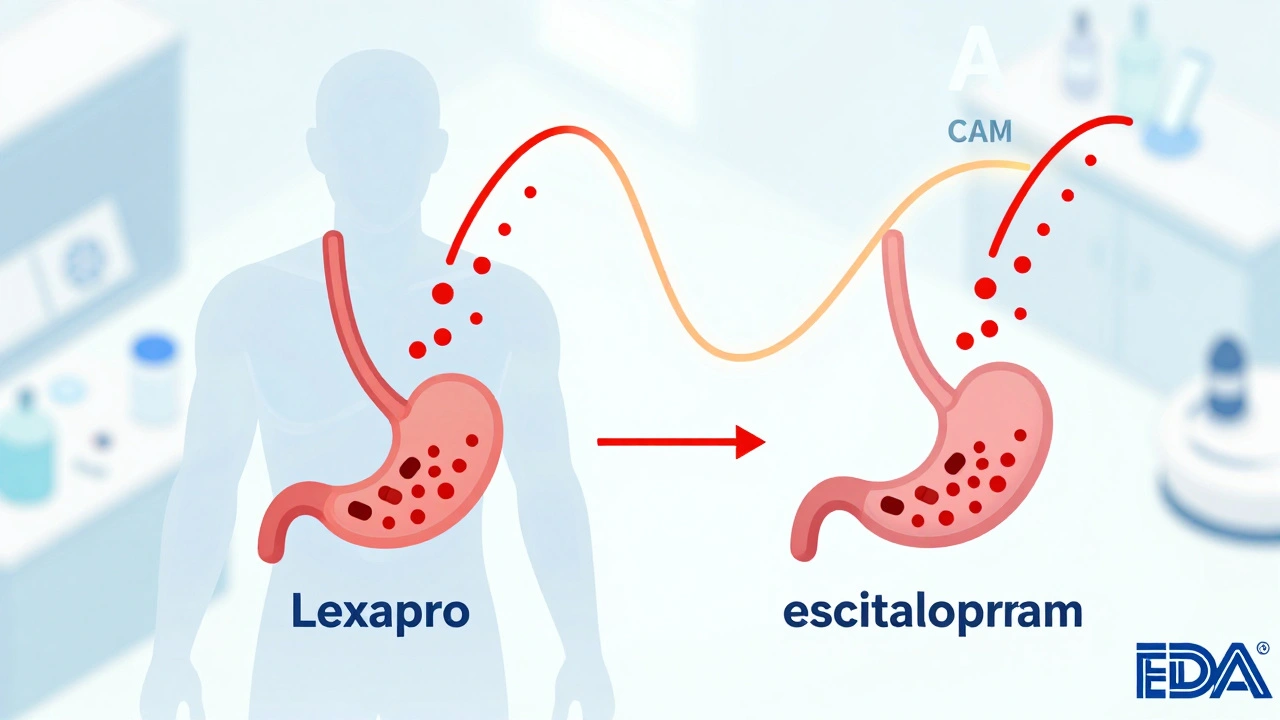
Clinical studies show that while most generic drugs work as well as brand-name versions, some patients - especially those on epilepsy or heart medications - face real risks when switching. Understanding when substitution is safe matters more than ever.
- 12
- Read More
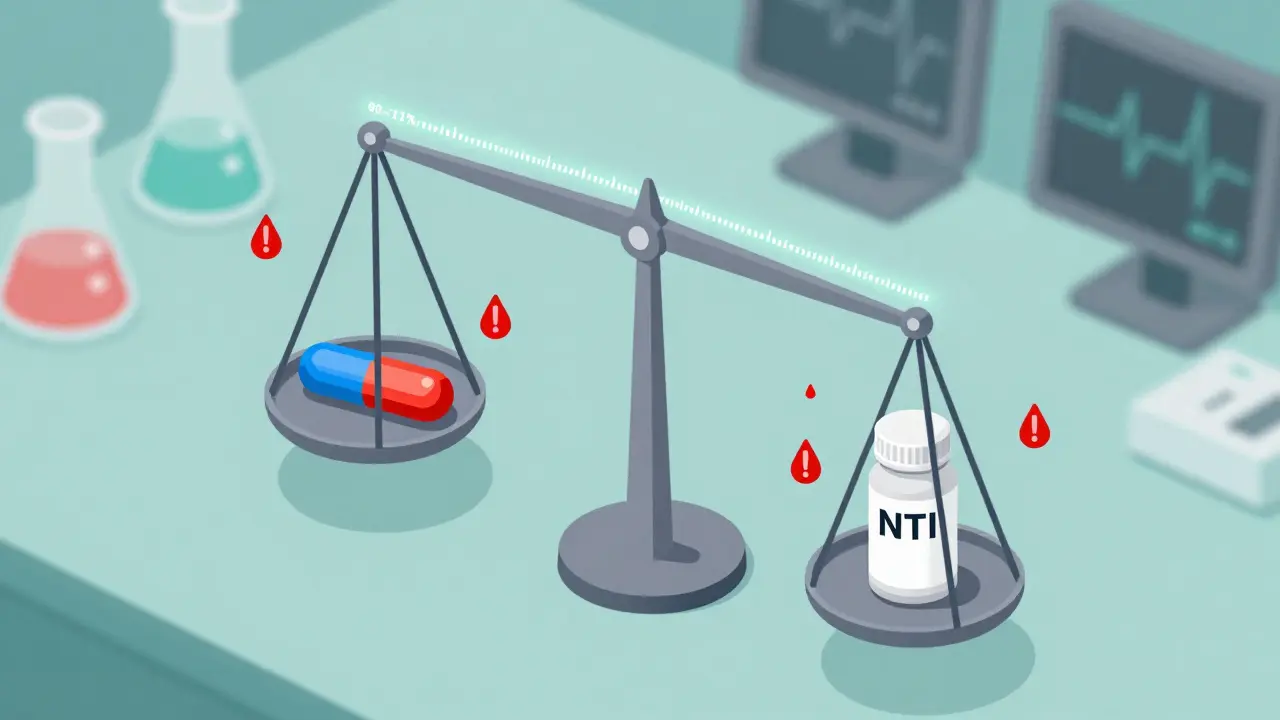
Bridging Studies for NTI Generics: Ensuring Safety and Efficacy
NTI generics require specialized bridging studies to ensure safety, as even small differences in absorption can lead to serious harm. Learn why these drugs demand stricter testing than standard generics and how regulators are adapting.
- 13
- Read More
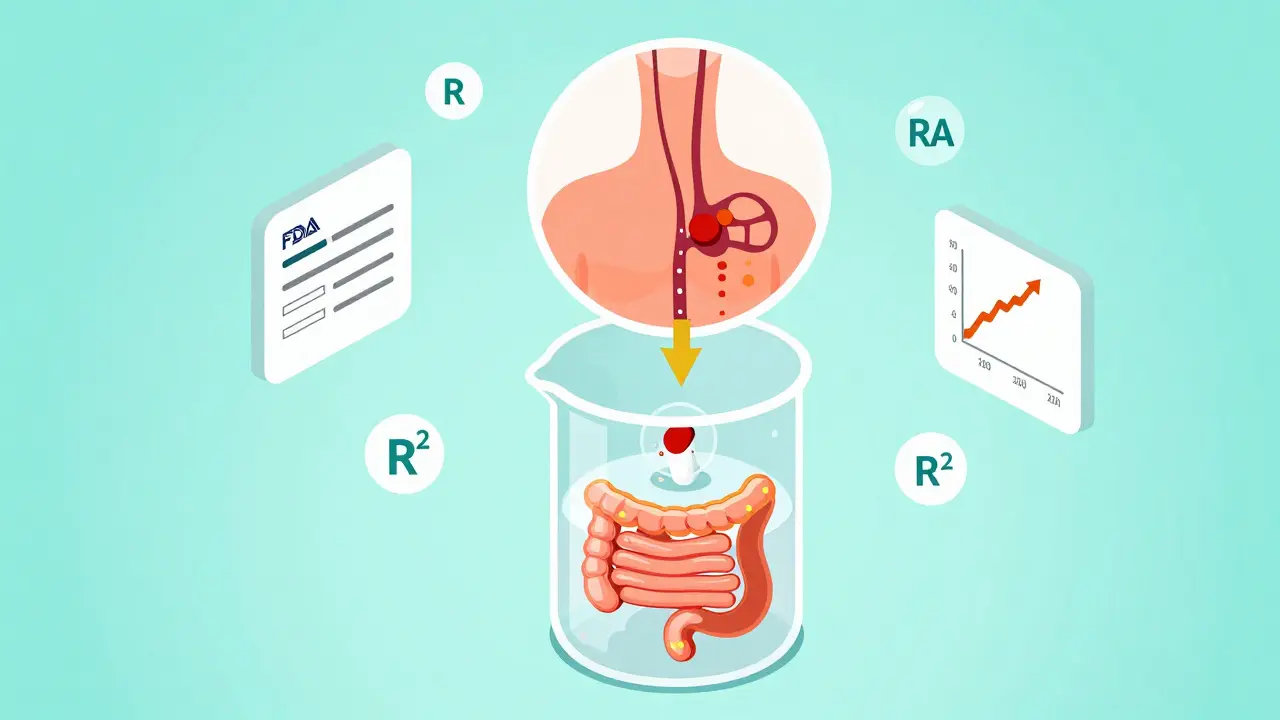
IVIVC and Waivers: How In Vitro Methods Are Replacing In Vivo Bioequivalence Testing
IVIVC uses lab-based dissolution testing to predict how drugs behave in the body, replacing costly human bioequivalence trials. Learn how it works, why most submissions fail, and why it's becoming essential for generic drug approval.
Bioequivalence and Patient Safety: Why Testing Ensures Safe Generic Medications
Bioequivalence testing ensures generic drugs work the same as brand-name versions, protecting patient safety while lowering costs. Learn how science keeps millions safe every day.
- 12
- Read More